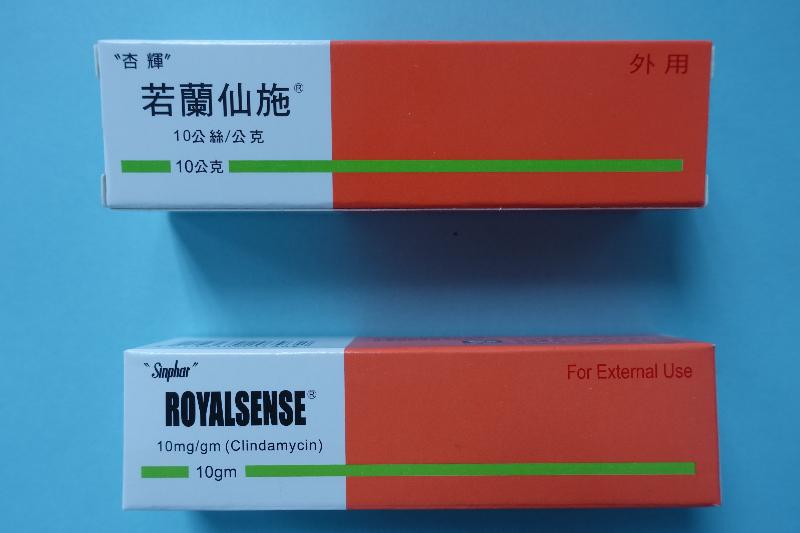
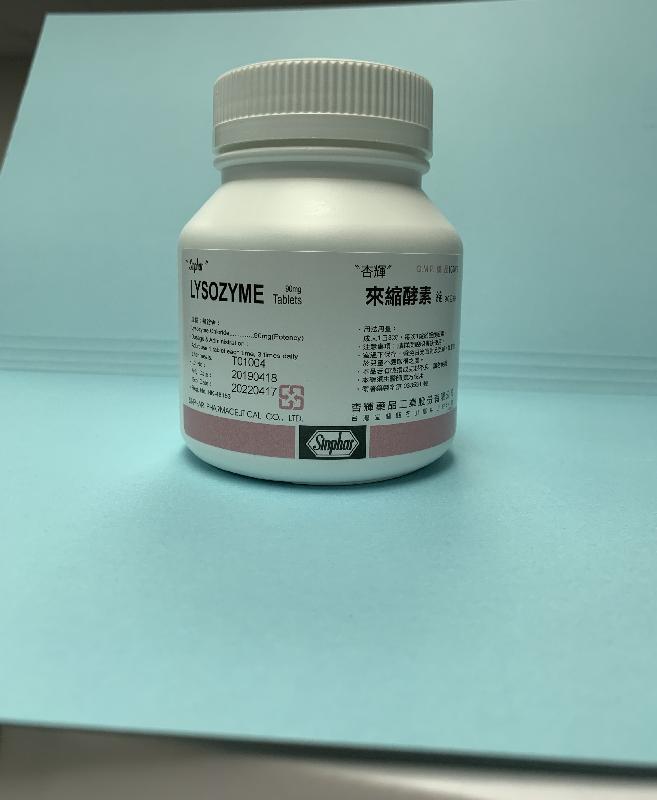
Recall of two pharmaceutical products (with photos)
The Department of Health (DH) today (September 14) endorsed a licensed drug wholesaler, Suntol Medical Ltd (Suntol), to recall Royalsense Acne Gel 1% (Hong Kong Registration Number: HK-48233) and Lysozyme Tablets 90mg (Sinphar) (Hong Kong Registration Number: HK-48153), from the market as a precautionary measure due to a stability issue.
The DH received notification from Suntol that Royalsense Acne Gel 1% and Lysozyme Tablets 90mg (Sinphar) are being recalled in Taiwan, as the content of active ingredients in the products failed to meet the product specifications during a long-term stability study. Though the products would not cause a health harm to users, the issue might affect the efficacy of the products. As a precautionary measure, Suntol is recalling the products in Hong Kong.
Royalsense Acne Gel 1%, containing clindamycin, is a prescription medicine used for the topical treatment of acne. Lysozyme Tablets 90mg (Sinphar), containing lysozyme, is an over-the-counter medicine used as adjunctive treatment for certain inflammatory conditions. According to Suntol, the products have been supplied to local private doctors and pharmacies.
Suntol has set up a hotline (2546 5699) to answer related enquiries.
"So far, the DH has not received any adverse reaction reports in connection with the products. The DH will closely monitor the recall," a spokesman for the DH said.
"Patients who are using the above products should seek advice from their healthcare professionals for appropriate arrangements," the spokesman added.