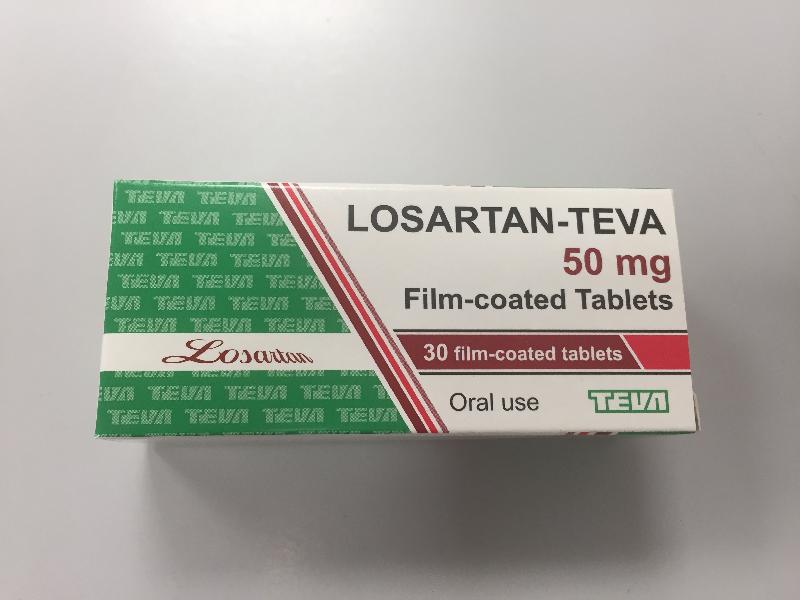
The Department of Health (DH) today (June 30) endorsed a licensed drug wholesaler, Teva Pharmaceutical Hong Kong Limited (Teva), to recall three batches (batch numbers: 0480918, 0681118, and 0760120) of Losartan-Teva Tablet 50mg (Hong Kong Registration Number: HK-58863) from the market as a precautionary measure due to the presence of an impurity in the product.
The DH received today notification from Teva of the finding by the overseas manufacturer of the product that the active pharmaceutical ingredient of the above batches contain a higher than accepted level of azido impurity. According to Teva, the three affected batches have been imported and supplied in Hong Kong. As a precautionary measure, Teva is voluntarily recalling those batches from the market.
Azido impurity is considered a mutagen that can cause a change in the DNA of a cell and may increase the risk of cancer, although the risk for the azido impurity to cause cancer in humans is unknown. Overseas drug regulatory authorities have been reviewing the safety impact of azido impurity found in medicinal products. The DH will closely monitor the development of the issue and any safety updates regarding the drug issued by overseas drug regulatory authorities for consideration of any action deemed necessary.
The above product is a prescription medicine used to lower blood pressure. According to Teva, the affected batches have been supplied to clinics of the DH, private clinics and community pharmacies.
Teva has set up a hotline (3904 3799) to handle related enquiries.
"So far, the DH has not received any adverse reaction reports in connection with the product. The DH will closely monitor the recall," a spokesman for the DH said.
"Patients who are taking the above product should not stop taking the medicine, but should seek advice from their healthcare professionals as soon as possible for appropriate arrangements," the spokesman added.
Follow this news feed: East Asia