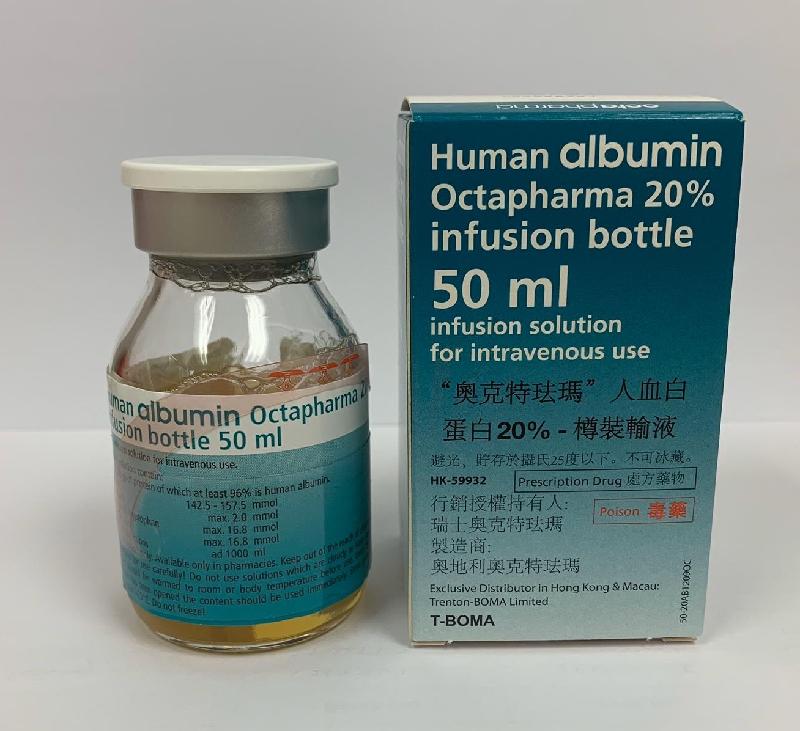
Recall of one batch of Human Albumin Octapharma 20% Infusion (with photo)
The Department of Health (DH) today (October 11) endorsed a licensed medicine wholesaler Trenton-Boma Ltd (Trenton-Boma) to voluntarily recall one batch (batch number: L828A6682) of Human Albumin Octapharma 20% Infusion (Hong Kong Registration Number: HK-59932) from the market due to a potential quality issue.
The DH received notification from Trenton-Boma today that the manufacturer of the product indicated that the production of the above batch has deviated from the processes approved by the European authority. Although the manufacturer considered that the quality of the product was not compromised, the company is recalling the affected batch as a precautionary measure.
The product, which contains human albumin, is a prescription medicine for plasma volume replacement. According to Trenton-Boma, the affected batch has been supplied to the Hospital Authority, private doctors and veterinary surgeons.
Trenton-Boma has set up a hotline (8101 2716) to answer related enquiries. The DH will closely monitor the recall.
"So far, the DH has not received any adverse reaction reports related to the product. People who have used the above product should consult healthcare professionals if in doubt," a DH spokesperson said.