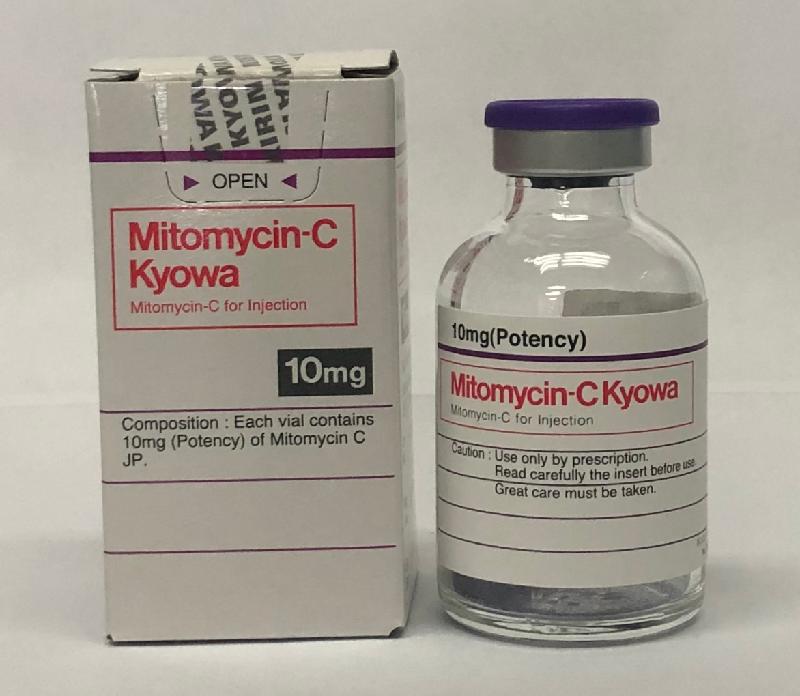
Recall of Mitomycin-C powder for injection products (with photos)
The Department of Health (DH) today (October 10) endorsed five licensed drug wholesalers, namely Tai Tong Co Ltd (Tai Tong); Four Seasons International Limited (Four Seasons); Trackcircle.com Limited (Trackcircle); Sino-Asia Pharmaceutical Supplies Ltd (Sino-Asia); and Vantone Medical Supplies Co Ltd (Vantone) to recall all Mitomycin-C powder for injection products from the market due to a potential quality issue.
The recall covers registered Mitomycin-C powder for injections as well as unregistered products imported by the companies for the treatment of particular patients. The affected products are listed below:
| Wholesaler | Product(s) | Hotline |
| Tai Tong | Mitomycin-C powder for injection 10mg KYOWA (Hong Kong Registration Number: HK-10969) Mitomycin-C powder for injection 2mg and 10mg KYOWA |
2544 6061 |
| Four Seasons | Mitomycin-C powder for injection 2mg and 10mg KYOWA | 3590 6313 |
| Trackcircle | Mitomycin-C powder for injection 10mg KYOWA | 3527 0198 |
| Sino-Asia | Mitomycin-C powder for injection 2mg KYOWA | 2573 2552 |
| Vantone | Mitomycin-C powder for injection 2mg KYOWA | 2601 2877 |
The DH received notification that the production of the active drug substance, i.e. mitomycin, of the above products did not fully comply with the Good Manufacturing Practice requirements and that the sterility of the substance could not be guaranteed. Although the products passed relevant tests and met the specifications, the companies are voluntarily recalling the products from the market as a precautionary measure.
The products, which contain mitomycin, are prescription medicines used mainly for the treatment of bladder cancer. According to the companies, the products were supplied to the local healthcare sector including the Hospital Authority, private hospitals and local doctors, while some have been exported to Macao. The companies have set up hotlines to answer related enquiries (see above table).
The DH will closely monitor the recall. So far, the department has not received any adverse reports in connection with the products concerned.
"People who have used the above products should consult their healthcare professionals if in doubt or feeling unwell," a DH spokesperson said.