Further recall of Eurogine SL intrauterine devices (with photos)
The Department of Health (DH) today (August 23) drew the public's attention to the further recall of intrauterine devices (IUDs) manufactured by Eurogine SL, due to an increased risk of breakage in the horizontal arms (one or both) of the IUDs at the time of extraction.
Further to the recall of two affected models involving five affected lots of IUDS by Eurogine SL announced on March 14, the DH received notification from the local supplier that additional lots of IUDs are being recalled. The additional affected lots are as follows:
| Model | Additional affected lots in Hong Kong |
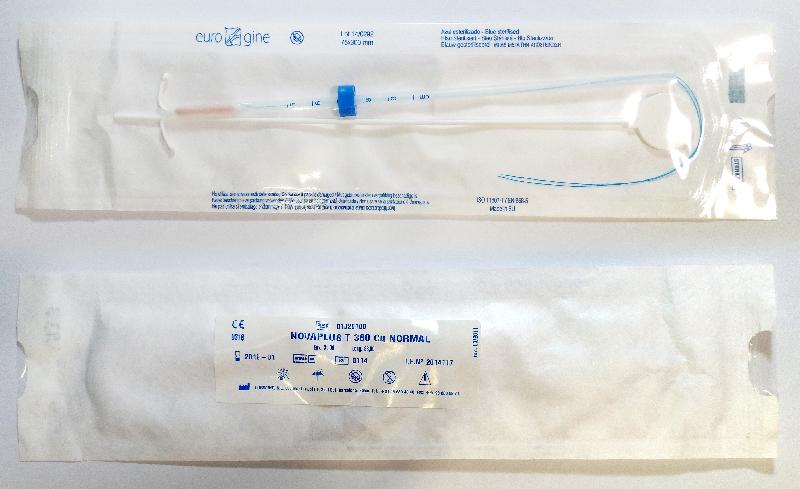
| Novaplus T380 Cu Normal | 1113, 0114, 0614, 0415 |
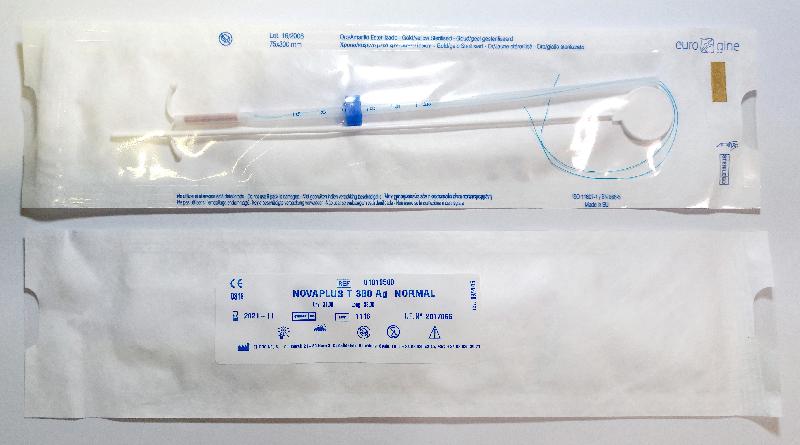
| Novaplus T380 Ag Normal | 0415 |
According to the manufacturer, the issue was caused by defective manufacturing of the raw material. The efficacy of the IUD is not affected and premature removal of the device is not recommended. When getting the IUD removed, it is recommended that the healthcare professional performs a slow and constant traction when pulling the threads.
According to the local supplier, around 1 700 units of the five affected lots of IUDs listed above were distributed to various organisations in Hong Kong, including private hospital, clinics and medical centres, as well as the Maternal and Child Health Centres (MCHCs) under the DH's Family Health Service. The supplier is notifying all affected organisations and replacement of the affected products is underway.
"The DH has informed relevant organisations about the recall and will continue to liaise with the local supplier on necessary follow-up actions. Women should consult the doctor who performed the IUD insertion, if in doubt," a spokesman for the DH said.
Regarding the affected IUDs with the DH, among the 1 000 affected units distributed to MCHCs, around 350 units have been used on clients attending family planning service. The DH will bring the matter to these clients' attention. Counselling and consultation will be offered if necessary.