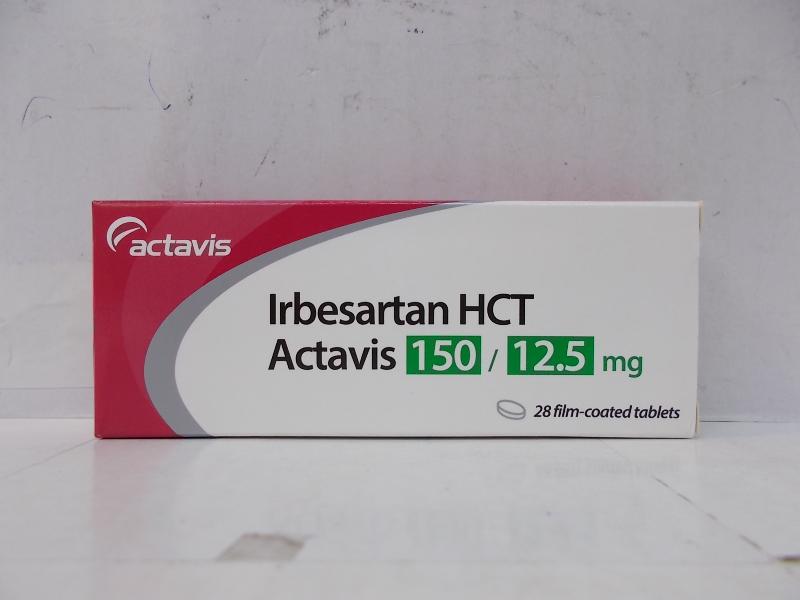
Batch recall of Irbesartan HCT Actavis Tablets 150/12.5mg (with photo)
The Department of Health (DH) today (December 20) endorsed a product registration certificate holder, Actavis Hong Kong Limited (Actavis), to recall one batch (batch number: 058818) of Irbesartan HCT Actavis Tablets 150/12.5mg (Hong Kong Registration number: HK-63378) from the market as a precautionary measure because an impurity was detected in one of the raw materials of this batch of product.
The DH received notification from Actavis today that, through its analytical testing, certain batches of the irbesartan raw materials were found to contain an impurity, N-nitrosodiethylamine (NDEA). NDEA is classified as a probable human carcinogen (a substance that could cause cancer). A number of irbesartan-containing products using these raw materials were thus affected. Among the affected products, the above batch of tablets has been imported into and supplied in Hong Kong. As a precautionary measure, Actavis has voluntarily recalled the affected batch from the market.
The above product, containing irbesartan and hydrochlorothiazide, is a prescription medicine used to lower blood pressure. According to Actavis, the affected batch has been supplied to local private doctors, pharmacies and one private hospital.
Actavis has set up a hotline (3188 4288) to answer related enquiries.
"So far, the DH has not received any adverse drug reaction report related to the affected product. The DH will continue its investigation and will closely monitor the recall," a spokesman for the DH said.
Patients who are taking the above product should not stop taking the medicine/s, but should seek advice from their healthcare professionals for appropriate management.