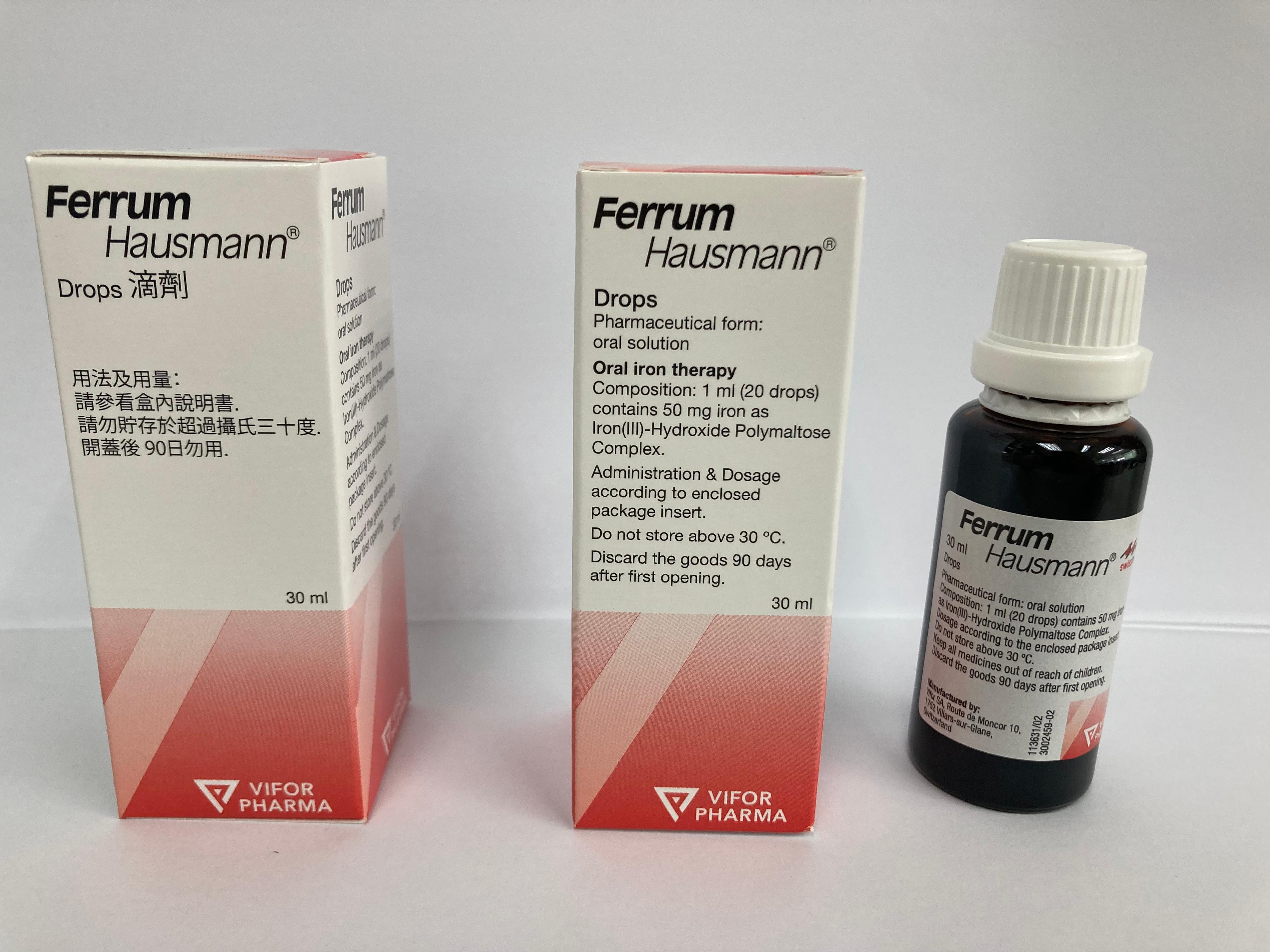
The Department of Health (DH) today (June 17) endorsed a licensed drug wholesaler, namely Hong Kong Medical Supplies Ltd (HKMS), to recall eight batches (batch numbers: AAL56301, AAM03804, AAP27103, AAR88304, AAT62402, AAW72104, NAA04302 and NAA14002) of Ferrum Hausmann Drops 50mg/ml (Hong Kong Registration Number: HK-36593) from the market as a precautionary measure due to a potential quality issue.
The DH received notification from HKMS that the overseas manufacturer of the product is initiating a voluntary recall of the above batches due to the presence of small plastic particles above the cap of the dropper in some samples of the product; the particles may fall out when used. As a precautionary measure, HKMS is voluntarily recalling the affected batches of product from the market. The DH's investigation is continuing.
The above product, containing iron, is an over-the-counter medicine for the treatment of iron deficiency and iron deficiency anaemia. According to HKMS, the concerned batches of product have been imported into Hong Kong for distribution to the Hospital Authority, local private hospitals, private doctors and pharmacies, as well as for re-export to Macao.
HKMS has set up a hotline (2806 3112) to answer related enquiries.
So far, the DH has not received any adverse drug reaction reports related to the affected batches of product. The DH will closely monitor the recall.
Members of the public should consult healthcare professionals if in doubt or feeling unwell after using the affected product.
Follow this news feed: East Asia