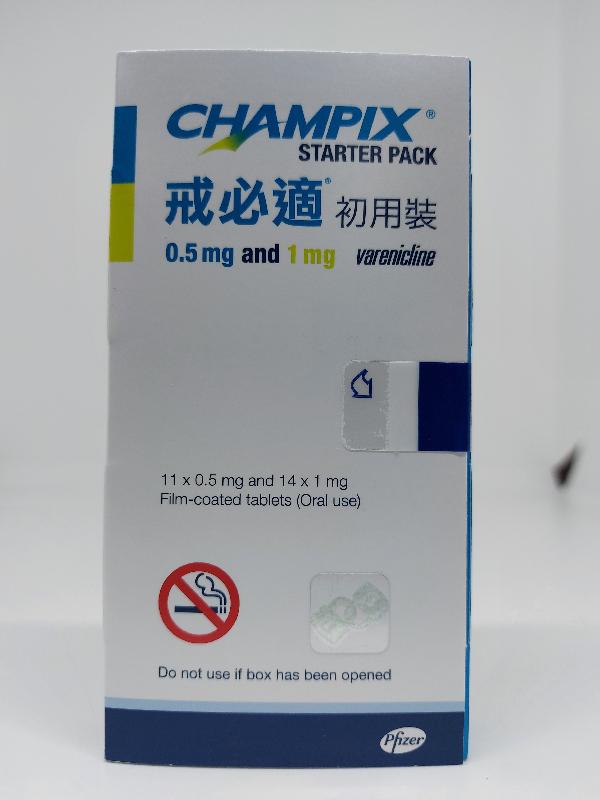
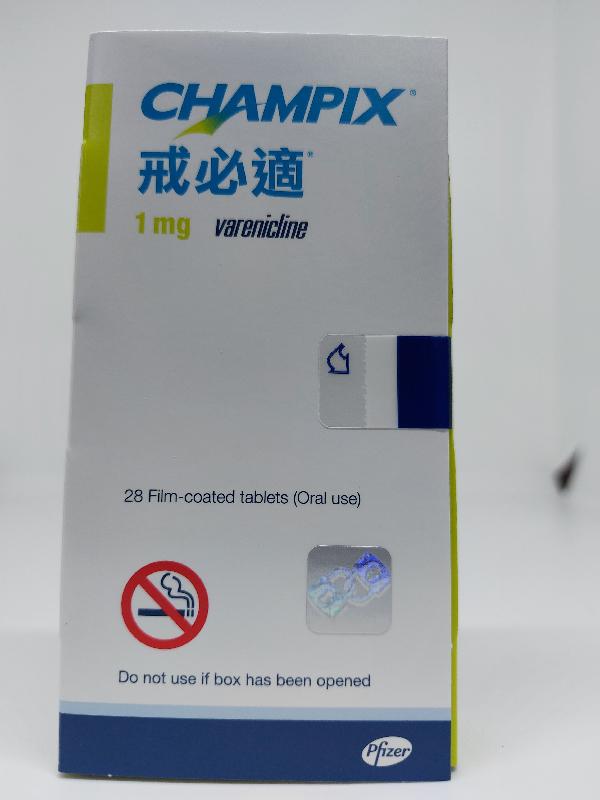
The Department of Health (DH) today (September 27) endorsed a licensed drug wholesaler, Pfizer Corporation Hong Kong Limited (Pfizer), to recall a total of five batches of the following two products from the market as a precautionary measure due to the presence of an impurity in the products.
| Name of product | Hong Kong registration number | Batch number |
| Champix tablets 0.5 mg and 1 mg | HK-55462 | 00023530 00026657 |
| Champix tablets 1 mg | HK-55437 | 00023023 00025603 00026556 |
The DH today received notification from Pfizer that the above batches of the products contain a nitrosamine impurity, namely N-nitroso-varenicline, exceeding the accepted level. The above batches are all currently available in Hong Kong. As a precautionary measure, Pfizer is recalling the above batches of the products from the market.
N-Nitroso-varenicline belongs to the nitrosamine class of compounds, some of which are classified as probable or possible human carcinogens, based on laboratory tests such as rodent carcinogenicity studies. Overseas drug regulatory authorities have been reviewing the safety impact of the nitrosamine impurity found in medicinal products. The DH will closely monitor the development of the issue and any safety updates regarding the drug issued by overseas drug regulatory authorities for consideration of any necessary follow-up action.
The above products contain the active ingredient varenicline and are prescription medicines used for smoking cessation. According to Pfizer, the products have been imported to Hong Kong and supplied to Hospital Authority hospitals, clinics of the DH, private hospitals, private doctors, community pharmacies, wholesalers as well as re-exported to Macao.
Pfizer has set up a hotline (2963 5514) to handle related enquiries.
"So far, the DH has not received any adverse reaction reports in connection with the products. The DH will closely monitor the recall," a spokesman for the DH said.
​"Patients who are taking the above products should not stop taking them, but should seek advice from their healthcare professionals as soon as possible for appropriate arrangements," the spokesman added.
Follow this news feed: East Asia